A genome-scale screen for synthetic drivers of T cell proliferation
Nature (2022)Cite this article
Abstract
The engineering of autologous patient T cells for adoptive cell therapies has revolutionized the treatment of several types of cancer1. However, further improvements are needed to increase response and cure rates. CRISPR-based loss-of-function screens have been limited to negative regulators of T cell functions2,3,4and raise safety concerns owing to the permanent modification of the genome. Here we identify positive regulators of T cell functions through overexpression of around 12,000 barcoded human open reading frames (ORFs). The top-ranked genes increased the proliferation and activation of primary human CD4+and CD8+T cells and their secretion of key cytokines such as interleukin-2 and interferon-γ. In addition, we developed the single-cell genomics method OverCITE-seq for high-throughput quantification of the transcriptome and surface antigens in ORF-engineered T cells. The top-ranked ORF—lymphotoxin-β receptor (LTBR)—is typically expressed in myeloid cells but absent in lymphocytes. When overexpressed in T cells, LTBR induced profound transcriptional and epigenomic remodelling, leading to increased T cell effector functions and resistance to exhaustion in chronic stimulation settings through constitutive activation of the canonical NF-κB pathway. LTBR and other highly ranked genes improved the antigen-specific responses of chimeric antigen receptor T cells and γδ T cells, highlighting their potential for future cancer-agnostic therapies5. Our results provide several strategies for improving next-generation T cell therapies by the induction of synthetic cell programmes.
Access through your institution Buy or subscribeThis is a preview of subscription content
Access options
Access through your institutionAccess through your institutionChange institutionBuy or subscribeSubscribe to Nature+
Get immediate online access to the entire Nature family of 50+ journals
$29.99
monthly
SubscribeSubscribe to Journal
Get full journal access for 1 year
$199.00
only $3.90 per issue
SubscribeAll prices are NET prices. VAT will be added later in the checkout.Tax calculation will be finalised during checkout.
Buy article
Get time limited or full article access on ReadCube.
$32.00
BuyAll prices are NET prices.
Data availability
Data from the ORF screen, OverCITE-seq, bulk RNA-seq and ATAC-seq have been deposited in the Gene Expression Omnibus (GEO) with accession number GSE193736. The following publicly available datasets have also been used in the study: DICE (https://dice-database.org/), Genotype-Tissue Expression Project v.8 (https://www.gtexportal.org/) and Single Cell Portal (https://singlecell.broadinstitute.org/single_cell/study/SCP424/single-cell-comparison-pbmc-data).

References
Download references
Acknowledgements
We thank the entire Sanjana laboratory for support and advice, and the New York University (NYU) Biology Genomics Core for sequencing resources. M.L. is supported by the Hope Funds for Cancer Research postdoctoral fellowship; Z.G. is supported by the National Institutes of Health (NIH) T32 Training Grant (GM136573); and N.E.S. is supported by NYU and NYGC start-up funds, NIH–NHGRI (R00HG008171 and DP2HG010099), NIH–NCI (R01CA218668), DARPA (D18AP00053), the Cancer Research Institute and the Sidney Kimmel Foundation.
Author information
Author notesAuthorsM.L. and N.E.S. designed the study. M.L. performed the screens and validation experiments (including single-cell and CAR work) and analysed data. Z.G. assisted with screens and single-cell data analysis. Z.G., M.G. and X.X. assisted with lentivirus production, T cell transduction, cell culture and flow cytometry. Z.D. and L.L. performed western blots. J.A.R. assisted with screen and ATAC-seq analyses. C.L. assisted with ATAC-seq. E.P.M., S.H. and P.S. assisted with single-cell experiments. T.D. contributed reagents. C.D. contributed patient samples. M.L. and N.E.S. wrote the manuscript with input from all authors. N.E.S. supervised the study.
Correspondence to Mateusz Legut or Neville E. Sanjana.
Ethics declarations
The New York Genome Center and New York University have applied for patents relating to the screening methods, identified targets and OverCITE-seq. C.D. receives research funding from Fate Therapeutics and Bristol Myers Squibb. N.E.S. is an adviser to Vertex and Qiagen.
Peer review
Nature thanks William Haining, Benedict Seddon and Elena Sotillo for their contribution to the peer review of this work.
Additional information
Publisher’s noteSpringer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
a, Barcoded vector design for ORF overexpression.b, Distribution of the number of barcodes per ORF in the library.c, Vector design for quantifying the effect of different promoters and ORF insert sizes on lentiviral transduction efficiency. EFS – elongation factor-1α short promoter, CMV – cytomegalovirus promoter, PGK – phosphoglycerate kinase-1 promoter.d, Sequential gating strategy and representative histograms of cells transduced with marker gene rat CD2 under different promoters.e, Percentage of positive cells andf, mean fluorescence intensity (MFI) of rat CD2 (rCD2) expressed from the EFS and CMV promoters, following puromycin selection of transduced primary CD4+ T cells. Each data point indicates individual transduction (n = 3 biological replicates). Error bars are SEM.g, Distribution of ORF sizes in the genome-scale library. The size of TCR-rCD2 construct tested in panels e and f is marked.h, Titration of CD3/CD28 antibodies. T cells were labelled with CFSE, stimulated and incubated for 4 days. Gate for proliferating T cells was set to include cells that proliferated at least twice (third CFSE peak).i, Expansion of T cells from three healthy donors transduced with the ORF library.j, Representative CFSE profile of restimulated CD8+ and CD4+ T cells before the sort. The CFSElowsort gate is marked.k, Recovery of individual barcodes or corresponding ORFs in transduced T cells and plasmid used for lentivirus production. Respective samples from three donors were computationally pooled together at equal number of reads prior to counting how many barcodes or ORFs were present with a minimum of one read.l, Distribution of reads corresponding to ORFs of different sizes. ORFs were assigned to ten quantiles based on their size, with Q1 being smallest size and Q10 being the largest size (n = 1,161 ORFs per quantile). Box shows 25–75 percentile with a line at the median; whiskers extend to 1.5 × interquartile range.m, Enrichment of genes in both CFSElowCD4+ and CD8+ T cells, calculated by collapsing individual barcodes into corresponding genes. Significantly enriched genes (log2fold change higher than 0.5 and adjusted p-value lower than 0.05) are marked in red. Immune response genes of interest are marked.n, Overlap of significantly enriched genes from panel m in individual screen populations (CD4+, CD8+) analysed separately.o, Normalized enrichment of individual barcodes for indicated genes in the CD8+ screen.p, GO biological processes for significantly enriched genes in panel m.q, Overlap of significantly enriched genes with differentially expressed genes between CD3/CD28 stimulated and naive T cells41.
a, Histograms of selected ORF expression in T cells after puromycin selection.b, Quantification of tNGFR expression in transduced CD4+ and CD8+ T cells. Puromycin selection was complete after 7 days post transduction. To maintain T cells in culture, they were restimulated with CD3/CD28 on days 21 and 42.c, Correlation between ORF sizes and changes in proliferation relative to tNGFR. Mean log2fold-changes are shown.d, Proliferation of restimulated CD8+ ore, CD4+ T cells relative to tNGFR in individual donors (n = 3 biological replicates). Mean and SEM are shown.f,g, Proliferation of T cells transduced with ORFs that significantly improved T cell proliferation (see Fig. 2c) measured by dilution of CellTrace Yellow. Representative CellTrace Yellow histograms and fitted distributions (f) as well as quantifications of the proliferation index (g) are shown (n = 3 biological replicates). P values: h, Viability of ORF-transduced T cells 4 days after CD3/CD28 restimulation. Representative data from one donor (out of 4 donors tested) are shown (n = 3 biological replicates).i,j, Cell cycle analysis of T cells stimulated with CD3/CD28 for 24 h. Gating was performed based on isotype and fluorescence minus one controls. Representative gating (i) as well as (j) quantification of cells in the S-G2-M phases (for stimulated T cells) are shown (n = 6 biological replicates from two donors). P values: 1, 0.29, 0.0065, 0.17, 0.0051, 1, 0.13, 0.55, 0.0004, 0.98, 0.0088, 0.68, 0.91, 0.7, 1 (left to right). Statistical significance for panelsgandj: one way ANOVA with Dunnett’s multiple comparisons test * p
a, Quantitative expression of CD25 or CD154 following restimulation. A minimum of two donors was tested in triplicate per gene. Only genes that significant increase T cell proliferation in CD4+, CD8+ or both T cell subsets are shown. Mean and SEM are shown.b,c, Sensitivity to antigen dose. T cells were incubated with indicated anti-CD3 antibody concentrations for 24 h and the amount of secreted IFNγ was quantified. Representative dose-response curve fitting (b) and IC50quantifications (c) are shown (n = 2 biological replicates).d, Quantification of secreted IL-2 and IFNγ in T cells incubated alone or with CD3/CD28 antibodies for 24 h. Representative data from one out of four donors (n = 3 biological replicates) are shown.e, Multiplexed quantification of selected secreted cytokines and chemokines by ORF-transduced T cells after 24 h of CD3/CD28 stimulation. Means of duplicate measurements (from independent samples) z-score normalized to tNGFR are shown. Absolute quantities of secreted cytokines and chemokines in stimulated and resting T cells are shown in Supplementary Table 7.
a, Quality parameters of cells as identified by gel bead barcodes. Negative, singlets and doublets are assigned based on cell hashing.b, Proportion of stimulated and resting T cells among cells assigned to each ORF. Chi-squared test p-values are shown for ORFs with significantly shifted (uneven) distributions of stimulated and rested cells.c, Cell-cycle corrected scaled expression of the overexpressed gene in the cells transduced with the respective ORF and negative control (tNGFR). Two-sided Wilcoxon test p-values shown above the violin plots indicate the statistical significance of gene expression level between specific ORF and tNGFR-transduced T cells. Box shows 25–75 percentile with a line at the median; whiskers extend to maximum and minimum values. N = 71 (ADA), 147 (AHCY), 190 (AHNAK), 119 (AKR1C4), 124 (ATF6B), 179 (BATF), 137 (CALML3), 189 (CDK1), 129 (CDK2), 236 (CLIC1), 84 (CRLF2), 91 (CXCL12), 88 (CYP27A1), 129 (DBI), 26 (DCLRE1B), 261 (DUPD1), 25 (FOSB), 119 (GPD1), 124 (GPN3), 199 (IFNL2), 60 (IL12B), 70 (IL1RN), 156 (ITM2A), 74 (LTBR), 88 (MRPL18), 167 (MRPL51), 107 (MS4A3), 69 (NFYB), 355 (NGFR), 261 (RAN), 182 (SLC10A7), and 56 (ZNF830) single cells.d, Expression of all ORF genes by cells assigned each ORF. Each row is z-score normalized.e, Distribution of individual ORF frequencies in clusters. Numbers of ORF cells and the chi-squared test residuals are displayed. Chi-squared test p-values indicating whether ORF distribution in each cluster significantly differs from overall ORF distribution are shown on top of the plot. Proportions of stimulated and resting T cells in each cluster are shown underneath the cluster label.f,g, Spearman correlations between transcriptional profiles of selected ORF cells in resting (f) and stimulated (g) populations.h, Fold change of top differentially expressed genes between cells with the indicated ORFs in resting and stimulated T cells. For each condition, the ORFs with the strongest transcriptional changes (compared to tNGFR cells) are shown.i, Differential gene expression in stimulated ORF T cells compared to resting T cells. Genes with significant expression changes in at least one ORF are shown (DESeq2 adjusted p 2 fold-change of each ORF (stimulated) to tNGFR (resting), normalized to log2fold-change of tNGFR (stimulated) to tNGFR (resting). Genes of interest in each cluster are labelled.j, Mean TCR clonotype diversity in ORF cells.
a, LTBR expression in the indicated human primary tissues from the Genotype-Tissue Expression (GTEx) project v875(n = 948 donors). Box shows 25–75 percentile with a line at the median.b, LTBR expression in peripheral blood mononuclear cells (PBMCs) from 31,021 cells from 2 donors76. Cell types indicated are derived from Harmony tSNE clustering of single-cell transcriptomes.c, Overlap between significantly upregulated genes in LTBR cells compared to tNGFR cells identified in single-cell or bulk RNA-seq.d,e, TCF1 expression in LTBR or tNGFR transduced T cells.d, Representative histograms of TCF1 expression and the gate for TCF1+ cells (dashed line) are shown, as well ase, quantification of TCF1+ cells (n = 3 biological replicates).f–h, ICAM-1, CD70, CD74, and MHC-II expression in LTBR and tNGFR T cells. Representative histograms (f), quantification (g) in n = 3 donors (CD8+) or n = 4 donors (CD4+) and time course (h) of expression in LTBR and tNGFR cells after CD3/CD28 stimulation (n = 3 biological replicates).i, Differentiation phenotype of NGFR and LTBR transduced T cells (n = 4 donors, CD4+ and CD8+ separately). CM: Central memory. EM: Effector memory. Differentiation was defined based on CD45RO and CCR7 expression (naïve: CD45ROnegCCR7+, CM: CD45RO+CCR7+, EM: CD45RO+CCR7neg, effector CD45ROnegCCR7neg).j, Representative dot plots of T cell viability after CD3/CD28 stimulation. Viable cells are in the lower left quadrant.k, Cell viability of CD4+ T cells transduced with LTBR or tNGFR lentivirus, either restimulated with CD3/CD28 for four days or left unstimulated (n = 2 donors with 3 biological replicates each).l,m, LTBR and tNGFR cells were stimulated with a 3:1 excess of CD3/CD28 beads every three days for up to three rounds of stimulation. Following repeated stimulation, expression of TIM-3 and LAG-3 (l) was measured in resting cells, and secretion of IFNγ and IL2 (m) was measured in restimulated cells (n = 3 biological replicates). Statistical significance for panelse,i, andk: two-sided unpaired t-test; for panelg: two-sided paired t-test. Error bars indicate SEM.
a, IL2 secretion after 24 h stimulation with CD3/CD28 antibodies. Where indicated, recombinant soluble LTA (1 ng/mL) or LIGHT (10 ng/mL) were added together with CD3/CD28 antibodies. CD4+ T cells from one donor were tested in triplicate.b,c, CD4+ and CD8+ T cells from two donors were co-incubated for 24 h with CD3/CD28 antibodies or recombinant soluble LTA or LIGHT and then IL2 (b) and IFNγ (c) were measured. (n = 3 biological replicates).d,e, Differentiation phenotype (d) or proliferation (e) after restimulation of tNGFR and LTBR transduced T cells (n = 3 biological replicates) incubated either with IL2 alone or with LTA (1 ng/mL) or LIGHT (10 ng/mL) for the duration of culture. CM: Central memory. EM: Effector memory. Unpaired two-sided t-test p values are shown.f–i, Transient LTBR or tNGFR expression via mRNA nucleofection (f). T cells were either nucleofected with LTBR or tNGFR mRNA (n = 3 biological replicates), and the surface expression of LTBR (g), tNGFR (h) or four genes upregulated in LTBR cells (i) was monitored over 21 days. At each timepoint the expression of target genes was normalized to matched tNGFR control.j, Schematic representation of FLAG-tagged LTBR mutants.k, LTBR and FLAG expression in T cells transduced with LTBR mutants. Error bars indicate SEM.
a, Principal component (PC) analysis of global accessible chromatin regions of LTBR and tNGFR T cells, either resting or stimulated with CD3/CD28 for 24 h.b, Differentially accessible chromatin regions between stimulated and resting tNGFR, stimulated and resting LTBR, resting LTBR and resting tNGFR, and stimulated LTBR and stimulated tNGFR. Numbers of peaks gained/lost are shown (using absolute log2fold change of 1 and adjusted p value c,d, Changes in chromatin accessibility (c) for differentially expressed (adjusted p d) for differentially accessible (adjusted p c) or genomic regions (d).e,f, Chromatin accessibility profiles at loci more (e) or less open (f) in LTBR compared to tNGFR cells, resting or stimulated for 24 h. The y-axis represents normalized reads (scale: 0–860 for BATF3, 0–1950 for IL13, 0–1230 for TRAF1, 0–1000 for TNFSF4, 0–300 for PDCD1, 0–2350 for LAG3).g, Chromatin accessibility in resting or stimulated LTBR and tNGFR cells. Each row represents a peak significantly enriched in LTBR over matched tNGFR control (log2fold change > 1, DESeq2 adjusted p value h, Correlations for each ATAC sample (biological replicate) based on the bias-corrected deviations.i, Top transcription factor (TF) motifs enriched in the differentially accessible chromatin regions in resting LTBR cells compared to resting tNGFR cells.
a, Phospho-RELA staining by intracellular flow cytometry in LTBR and tNGFR cells. Gating for identification of phospho-RELA+ cells is shown.b,c, Western blot quantification of key proteins in the NF-κB pathway in LTBR and tNGFR cells, resting or stimulated with CD3/CD28 for 15 min. Representative gels (b) or quantification of band intensity relative to GAPHD (c) are shown (n = 3 biological replicates). Unpaired two-sided t test p values are shown.d, Representation of the LTBR signalling pathway. Each gene is coloured based on the differential expression in LTBR over matched tNGFR cells (CD4+ and CD8+ T cells, resting or stimulated for 24 h).e–g, Simultaneous gene knockout via CRISPR and ORF overexpression. T cells were transduced with a lentiviral vector co-expressing a single guide RNA (sgRNA) and the LTBR ORF. After transduction, Cas9 protein was delivered via nucleofection.f, Representative expression of target genes in LTBR cells co-expressing an sgRNA targeting B2M, an essential component of the MHC-I complex, or TRBC1/2, an essential component of the αβ TCR.g, Quantification of IFNγ after restimulation (n = 3 sgRNAs).h–o, Representative protein-level based quantification of gene knockout efficiency. Representative histograms (h,j,l) and quantification of relative expression levels of LTA, LIGHT, and RELA (i,k,m) are shown (n = 3 sgRNAs). Dashed lines represent gates used to enumerate cells expressing a given protein. Representative gel (n) and quantification of RELB expression (o) are shown (n = 3 sgRNAs for RELB and 2 non-targeting control sgRNAs).p, Identification of 274 genes identified as enriched in both CD4+ and CD8+ T cells transduced with LTBR over matched tNGFR controls (“core LTBR” genes). See Supplementary Fig. 1 for uncropped gel images. Error bars indicate SEM.
a, Transduction efficiency of CAR+ORF lentiviral vectors or ORF alone (n = 4 biological replicates).b,c, CAR expression level as determined by staining with anti-mouse Fab F(ab’)2. Representative histograms (b) and quantification of CAR expression relative to tNGFR (c) is shown for two healthy donors and two patients with diffuse large B cell lymphoma (DLBCL).d, Expansion curves of CAR+ORF transduced T cells (n = 4 biological replicates).e, LTBR expression in autologous CD14+ monocytes and T cells transduced with LTBR alone or CAR+LTBR.f–I, Expression of ICAM-1 (f), CD70 (g), CD74 (h) and MHC-II (i) by T cells transduced with LTBR ORF only, CAR + LTBR or CAR + tNGFR. All data are normalized to tNGFR only (no CAR). Unpaired two-sided t test p values are shown.j–m, Expression of exhaustion markers PD-1 (j), TIM-3 (k), LAG-3 (l) and CD39 (m) in CAR+ORF T cells.n, Differentiation phenotype of CAR+ORF T cells. CM: Central memory. EM: Effector memory. Differentiation was defined based on CD45RO and CCR7 expression (naïve: CD45ROnegCCR7+, CM: CD45RO+CCR7+, EM: CD45RO+CCR7neg, effector CD45ROnegCCR7neg).o,p, Expression of activation markers CD25 (o) and CD69 (p) in CAR+ORF T cells incubated alone or with Nalm6 cells for 24 h. Error bars indicate SEM. N = 3 biological replicates, unless indicated otherwise.
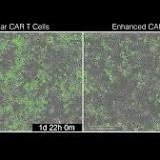
a,b, Co-delivery of anti-CD19 CARs and ORFs to T cells from healthy donors.a, IFNγ andb, IL2 secretion after overnight co-incubation of CD4+ T cells with Nalm6 cells at 1:1 ratio (n = 3 biological replicates, representative of two donors).c,d, IFNγ (c) or IL-2 (d) secretion by CAR+ORF or ORF only T cells co-incubated for 24 h either alone or with Nalm6 cells.e, Cytotoxicity of 19-BBz CAR T cells expressing tNGFR or LTBR ORF after co-incubation with Nalm6 GFP cells.f, Quantification of Nalm6 clearance (relative to Nalm6 co-incubated with untransduced T cells) for CAR+ORF or ORF alone T cells at different effector:target ratios. Unpaired two-sided t-test p values: 0.011, 1.3x10−4, 0.072, 0.02, 0.021, 0.52, 0.087, 1, 0.51 (left to right).g, Representative images of T cells transduced with 19-28z CAR and NGFR or LTBR, co-incubated with CD19+ Nalm6 GFP cells for 48 h at 1:1 ratio. Scale bar: 200 μm.h–j, Repeated stimulation of CAR+ORF T cells with Nalm6 cells. IL-2 secretion (i), or Nalm6 survival (j), by 19-BBz CAR LTBR or tNGFR T cells re-challenged with Nalm6 after repeated stimulation with Nalm6 cells every three days, for up to three rounds of stimulation.k, Secretion of cytokines IL2 and IFNγ by CAR/LTBR or CAR/tNGFR T cells from two patients with DLBCL after overnight incubation with Nalm6 target cells. Two-sided paired t-test p value is shown.l, Representative staining of ORF-transduced T cells endogenously expressing Vγ9Vδ2 TCR.m, Quantification of ORF-transduced T cells expressing Vγ9Vδ2 TCR.n,o, IL2 (n) or IFNγ (o) secretion after 24 h co-incubation of ORF transduced Vγ9Vδ2 T cells with leukaemia cell lines.p, IL2 or IFNγ secretion after 24 h co-incubation of ORF transduced Vγ9Vδ2 T cells with BxPC3, a pancreatic ductal adenocarcinoma cell line. Cell lines in panelsn–pwere pre-treated with zoledronic acid prior to co-incubation. Error bars indicate SEM. N = 3 biological replicates are shown, unless indicated otherwise.
Supplementary information
This file contains Supplementary Figure 1 (source gel images for western blot analyses) and the legends for Supplementary Tables 1–16 (see separate Excel file for the Supplementary Tables).
This file contains Supplementary Tables 1–16 (see Supplementary PDF for table legends).
Rights and permissions
Reprints and Permissions
About this article
Legut, M., Gajic, Z., Guarino, M. et al. A genome-scale screen for synthetic drivers of T cell proliferation.Nature(2022). https://doi.org/10.1038/s41586-022-04494-7
Download citation
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
}})