How a game-changing transplant could treat dying organs
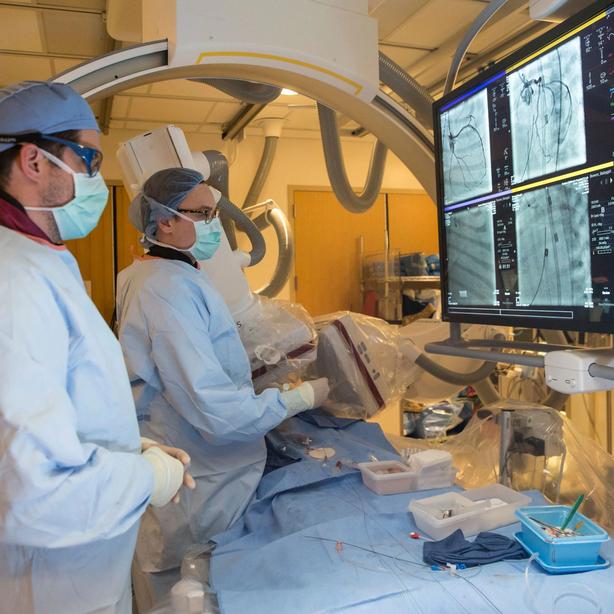
Doctors started preparing her for a heart transplant, but they noticed that during the brief moments when they disconnected her from ECMO, the machine that pumped her blood, in order to clean the tubes, her heart was functioning a bit better than they’d expected, suggesting the organ might be salvageable. That’s when Sitaram Emani, a cardiovascular surgeon and department head at Boston Children’s Hospital, approached Blias and offered to perform an experimental procedure to save her daughter’s life: a mitochondrial transplant.
This procedure involves collecting a patient’s mitochondria—tiny oval structures that provide cells with energy to function—and injecting them into damaged tissue. If it works as expected, the healthy mitochondria get absorbed into damaged cells and help them heal from the inside. With no other options for the infant Avery, “It was kind of like a Hail Mary,” Blias says. Amazingly, it worked. Avery’s heart pumped stronger each day, and she was able to go home. She’s had a total of six heart surgeries in as many years and still needs regular cardiological treatment, but if you saw her today, you’d never know anything was amiss.
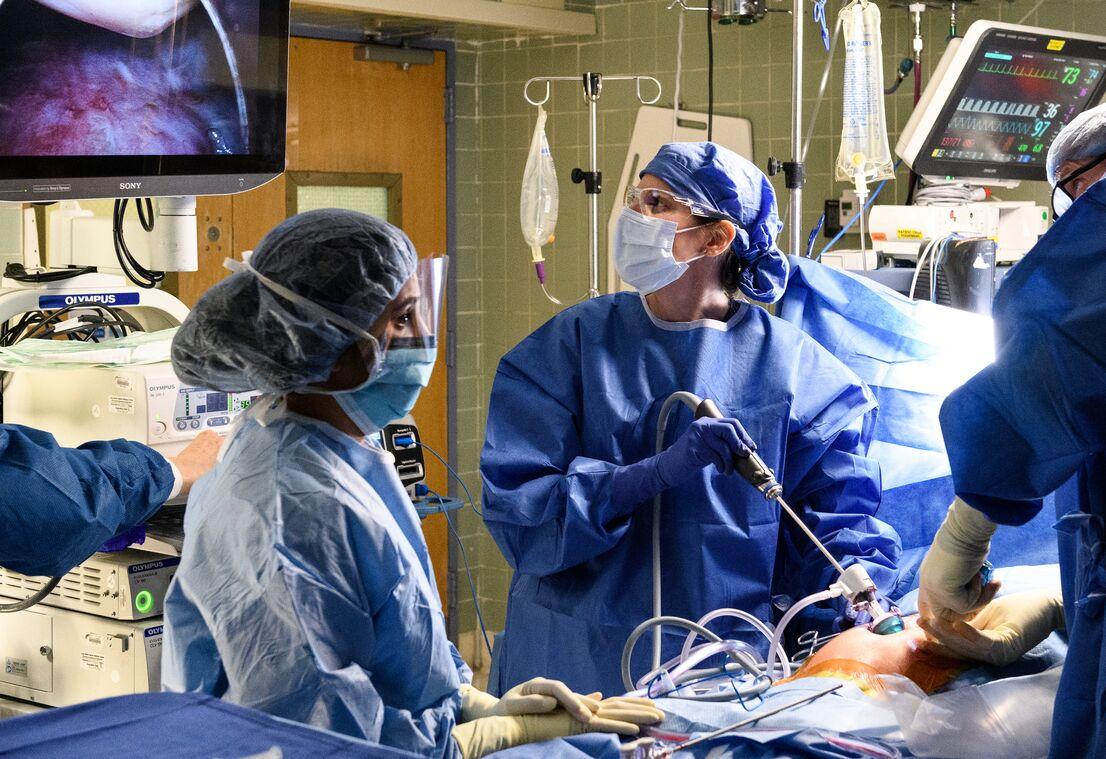
Now scientists are betting that an infusion of mitochondria will jump-start the cellular processes required to heal damaged hearts, brains, and maybe even other organs in a way that drugs haven’t been able to. So far, the results from animal models and a few first-in-human trials like Avery’s have been promising. In the past few years new biotech companies have launched to harness the power of mitochondria for applications from wound healing to anti-ageing.
There’s still a lot left to learn, and at this stage there is little government funding for this type of research. The Boston team, for instance, relies heavily on philanthropic donations. Michael Levitt, an associate professor of neurological surgery at the University of Washington, is working to transplant mitochondria into the brains of stroke patients, and he says his team has “no external funding whatsoever. This is all blood, sweat, and tears.”
Still, the scientists following this path are hopeful mitochondrial transplants will be a game-changer for treating a range of conditions, from wounds to strokes and heart attacks. “We’re so optimistic about what this could mean,” says Melanie Walker, a clinical professor of neurological surgery at the University of Washington and a colleague of Levitt’s.
Mitochondrial mayhem
Mitochondria are often described as “the powerhouses of the cell” because they manufacture a molecule known as adenosine triphosphate, or ATP. This molecule stores energy from the food you eat and uses it to fuel activities in different parts of your cells.
Scientists have long known that defective mitochondria can cause biological chaos. “Mitochondrial dysfunction is a universal driver of disease,” says Keshav Singh, a mitochondria researcher at the University of Alabama Birmingham who founded the Mitochondria Research and Medicine Society in the U.S. and India. Whether tissue damage is caused by disease or even space travel, faulty mitochondria are frequently involved.
}})